The radiologist as a healthcare navigator
Interview with Prof. Hildo J. Lamb, M.D., Professor of Radiology and Director of Cardiovascular Imaging at Leiden University Medical Center.
Professor Lamb, how long have you been working with SEARCH Lung CT?
Prof. Dr. Hildo J. Lamb: We first started talking to contextflow about three years ago. This then turned into a partnership. We have been working together intensively for about two years and continue to develop the solutions together.
How should I understand this cooperation?
Prof. H. J. Lamb: One of the challenges in radiology, for example, is the integration of AI solutions into reporting systems. We work with a system from Sectra that is open to the integration of third-party systems. We have thus managed to integrate contextflow’s AI solution into the workflow and are now using it in routine clinical practice.
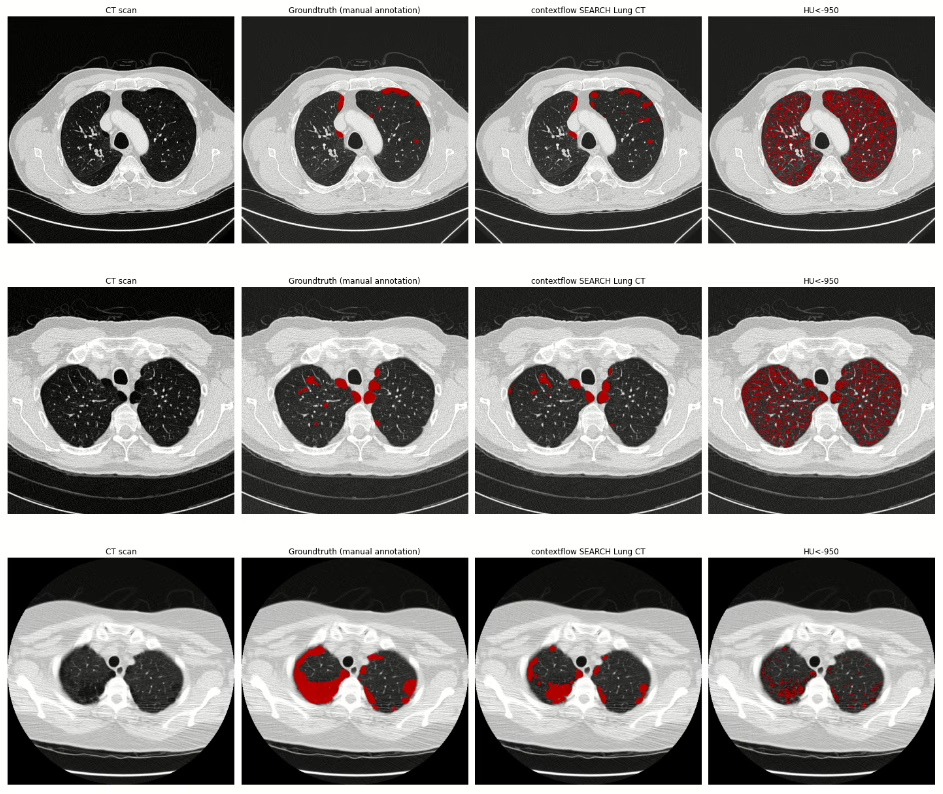
We are currently working with SEARCH Lung CT and want to use the software to identify abnormal findings in CT images. We expect this to lead to better workflow management. In the morning, we start well-rested and fresh with abnormal, complex cases. In the afternoon, when a certain fatigue sets in, the cases classified as normal by the artificial intelligence (AI) are then assessed. We hope this approach will result in safer and better reporting.
Are there already further plans with SEARCH Lung CT?
Prof. H. J. Lamb: Oh yes. When all radiologists are convinced that this AI solution will support them effectively, we will use it in the long-term, for example, to calculate lung nodule volume or volume doubling times. That will be another leap in quality. Currently, due to time constraints, we only look at the last preliminary scan. It’s impossible to manually quantify all the scans over the course of a lung cancer treatment. AI can do that. With all the automated tools, we can create a curve from each data point and track the change in nodule volume. Today, we only measure the diameter of the lesions. But it is better to look at the volume because that is much more sensitive to changes over time. That should be the next step with SEARCH Lung CT.
You talk a lot about therapy management and your roles in that. Where do you see the role of the radiologist in the future?
Prof. H. J. Lamb: We are faced with the challenge that both the image data volume of the images and the complexity of the cases are constantly increasing. At the same time, the need for quantification – here in particular of volumes, less so of diameters – is increasing because we cannot evaluate lesions visually alone. All in all, this leads to an exponential increase in our workload. The dangers range from incorrect reporting to burnout. I am convinced that AI can help us immensely. It is predestined to detect lesions and quantify them automatically. Importantly, we still need a radiologist to interpret this quantification. This is exactly where I see the future of our discipline: in the interpretation of findings, in explaining the results against the background of the patient’s entire medical history, and in advising on the next steps.
In other words, the radiologist as a pilot.
Prof. H. J. Lamb: We actually see the radiologist as a navigator of health care. We want to be the spider in the web of healthcare, with an integrated view of the patient. So we’re not just looking at the heart or just the lungs or just the brain, but the whole body. We need to leave behind specialization and niche thinking and look at the patient as a whole. To do this, however, we need time, and AI-supported systems can give us that by taking over time-consuming routine activities.
How far away is this scenario?
Prof. H. J. Lamb: It’s hard to say. First of all, we have to develop all the necessary technologies. Then they have to be reliable and validated so that they work smoothly. That will certainly take a few more years. I think that in five years we will have some kind of basic arsenal of techniques to do this. And then the transition has to start, that we embrace it and accept the integration of AI.
What impact will AI have on the work of the radiologist then?
Prof. H. J. Lamb: The workflow for radiologists is going to change over the next five to 10 years. That is why it is so important that we get involved now, at the beginning of this change. We can’t just wait and see what the engineers and companies do. Rather, we need to take the lead in this change. We need to define what our future looks like.
That brings us back to the topic of collaboration with companies. What should this look like?
Prof. H. J. Lamb: The best model we have experienced is to develop a product together in co-creation. If a solution is designed in an ivory tower, it will never meet our requirements. The key to successful and accepted AI solutions therefore lies in close cooperation between users and companies.
Thank you very much for the exciting and inspiring insights, Professor Lamb.